Your data. Your AI.
Your future.
Own them all on the new data intelligence platform

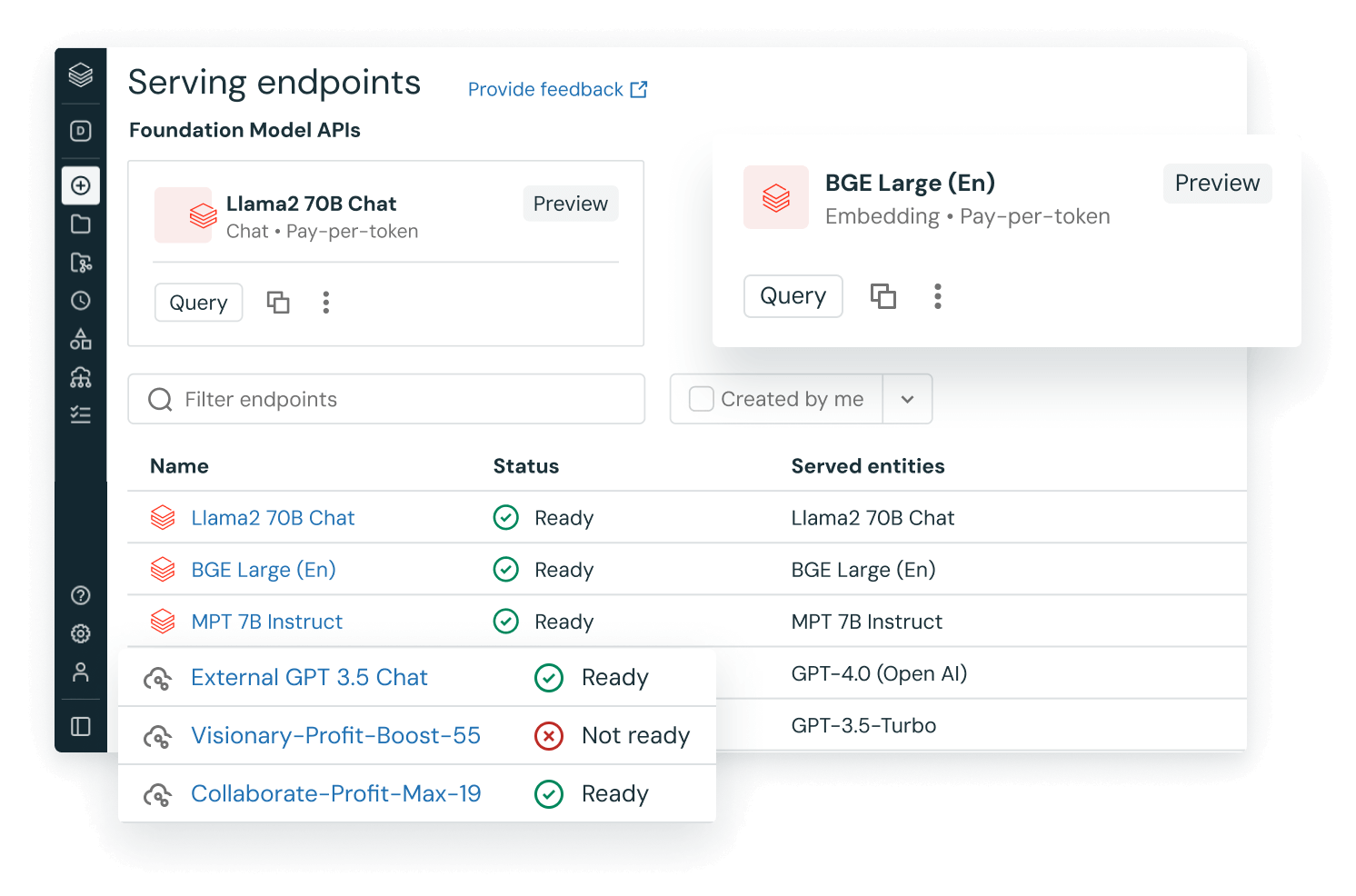
Meet DBRX, the new standard for high‑quality and efficient LLMs
DBRX, our new, open source foundation model, sets the standard for quality and efficiency. DBRX outperforms all established open models in quality benchmarks and allows you to quickly build your own custom LLM on your data.
The Databricks
Data Intelligence Platform
Databricks brings AI to your data to help you bring AI to the world.Unify all your data + AI
Industry leaders are data + AI companies
Plug into what you already use
Speed up success in data + AI
The Databricks Data Intelligence Platform integrates with your current tools for ETL, data ingestion, business intelligence, AI and governance. Adopt what’s next without throwing away what works.
More than meets the AI
In the spotlight
Ready to become a data + AI company?
Take the first steps in your transformation